Building a Scalable LIS for Toxicology Testing Labs
Why Toxicology Labs Have Unique Scaling Challenges
Toxicology testing labs are built for precision but as testing volume increases, so do operational demands. From managing high-throughput urine drug screening to ensuring strict chain-of-custody protocols, growing toxicology labs face a different set of challenges than general clinical labs.
The more complex your workflow becomes, the more your Laboratory Information System (LIS) becomes a bottleneck unless it’s built to scale.
A scalable LIS is not just about supporting more samples. It’s about maintaining accuracy, auditability, and speed as your operation grows. Whether you’re expanding to new locations, adding new test panels, or onboarding more clients, your software should grow with you not against you.
What Scalability Means in the LIS Context
A scalable LIS adapts to increased demand without sacrificing performance or oversight. That means:
- Supporting more concurrent users and workstations
- Automating repetitive steps like result entry and report generation
- Managing a growing list of tests, cut-offs, and client-specific formats
- Enabling faster turnaround time, even with high sample volumes
- Remaining compliant with CLIA, CAP, and lab-specific SOPs
- Integrating smoothly with external systems like analyzers, EMRs, and billing platforms
Without scalability, growth leads to delays, errors, and frustrated staff. With the right LIS, it leads to efficiency, confidence, and opportunity.
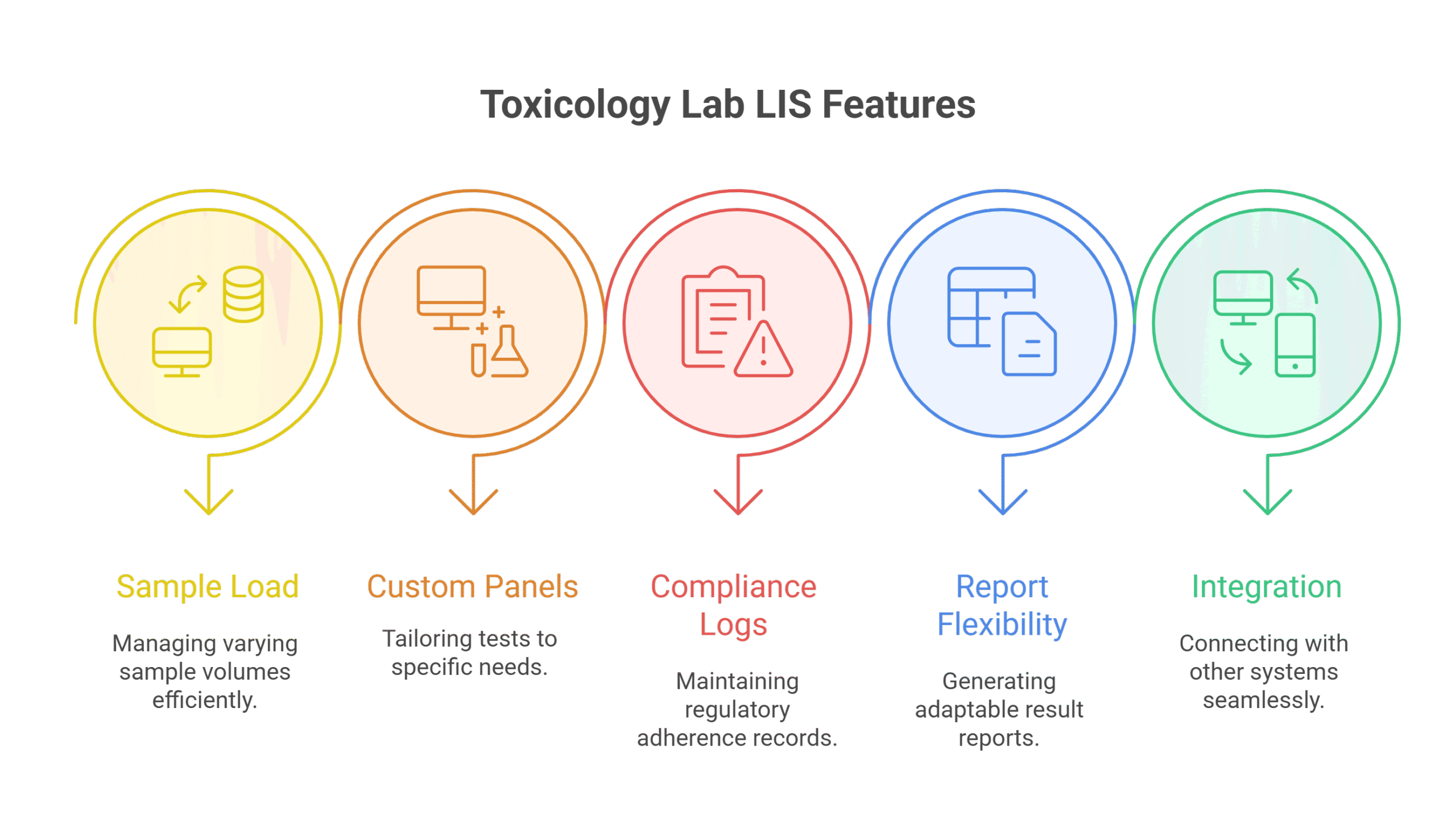
Core LIS Features Toxicology Labs Need to Scale
High-Volume Sample Management
Toxicology labs often process hundreds or thousands of samples daily. Your LIS should allow:
-
- Batch accessioning and barcode generation
- Sample tracking from intake to disposal
- Load balancing across instruments and staff
- Real-time dashboard monitoring of sample progress
Prolis helps labs streamline this with intelligent workflow design and scalable infrastructure.
Configurable Test Panels and Cut-Offs
Each toxicology client may have unique test panels, reporting thresholds, or detection windows. A scalable LIS supports:
-
- Custom panels for each client or test type
- Configurable cut-off levels per analyte
- Reflex testing rules
- Flagging of abnormal or diluted specimens based on lab rules
With Prolis, these can be managed without custom code just configuration.
Chain of Custody and Audit Trails
For legal and occupational testing, chain-of-custody compliance is critical. Prolis supports:
-
- Digital signature capture
- Time-stamped custody tracking
- Secure, read-only audit logs for all interactions
- Role-based access to custody documentation
Labs using Prolis stay inspection-ready with complete traceability at every handoff.
Custom Result Templates and Interpretive Reporting
Clients need more than raw numbers. Toxicology labs must deliver clear, customizable, and legally sound reports.
Prolis offers:
-
- Branded result templates per client
- Integrated interpretive comment logic
- Auto-release or supervisor-approval workflows
- Support for PDF, HL7, and web-portal formats
This helps labs meet each client’s expectations without slowing the lab down.
Integration with EMRs, Billing, and Analyzers
As your lab grows, you’re likely working with more external systems. A scalable LIS must handle:
-
- Analyzer interfaces for qPCR, GC/MS, LC/MS, and immunoassay platforms
- Bi-directional HL7 interfaces with EMRs
- Seamless claim generation and billing rule application
- Real-time AR and claim tracking
Prolis connects with the full lab ecosystem, enabling automation across departments and platforms.
Signs Your Current LIS Is Holding You Back
If your toxicology lab is growing but your LIS isn’t, you may notice:
- Frequent delays in reporting or verification
- Manual workarounds for client-specific needs
- Limited customization options
- Errors in chain-of-custody documentation
- Difficulty integrating new instruments or tests
- Billing complications due to LIS data gaps
These are red flags that your system isn’t scalable and could be costing you more than just time.
How Prolis Supports Growth in Toxicology Workflows
Prolis is built for volume, complexity, and customization. For toxicology labs, it provides:
- Configurable templates for each stage of the workflow
- High-performance architecture that supports rapid growth
- Tools for compliance documentation and audit prep
- Role-based dashboards for workload balancing
- Full-scale support for integrations and interfaces
Whether you’re serving five clinics or fifty, Prolis gives you the foundation to expand with confidence.
Grow Your Lab with the Right System
In toxicology, growth should not mean more manual work, more errors, or more compromises. With the right LIS, it means more clarity, more automation, and more success.
If your lab is scaling or ready to start Prolis delivers the tools to help you grow without slowing down.